Clinical context
Toxoplasmosis
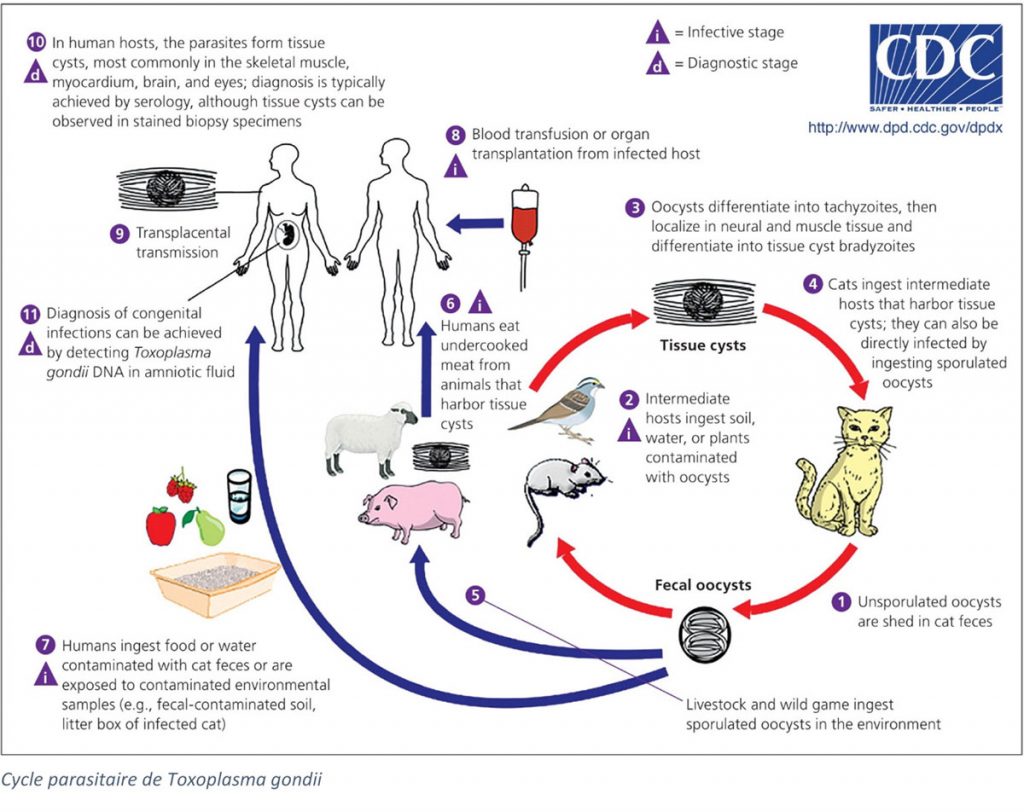 Toxoplasmosis is a zoonosis caused by the parasite Toxoplasma gondii. This obligate intracellular protozoan has a complex cycle, involving several stages:
Toxoplasmosis is a zoonosis caused by the parasite Toxoplasma gondii. This obligate intracellular protozoan has a complex cycle, involving several stages:
- A sexual cycle in felines, final hosts. The parasite reproduces during the merozoite stage in cats, which then releases the oocyst form of the parasite in its droppings: this is the sporozoite stage.
- An asexual cycle in intermediate hosts, mammals including humans, and birds. The parasite proliferates in vacuoles in the host during the tachyzoite stage, before infecting any type of nucleated cell, where it will be enclosed in cysts by several thousand: bradyzoite stage.
If T. gondii most likely originated in the Amazon (Reiling et al., 2019), toxoplasmosis is the most common parasitic disease in the world, with about 30% of the world’s population infected. This number hides important regional disparities (Pappas et al., 2009). Prevalence has been declining in recent years, particularly in Western countries, probably due to improved hygienic conditions (Guigue et al., 2018).
Humans become infected by ingesting cysts from raw or undercooked meat, or oocysts from water or food contaminated with the droppings of infected cats. Infection can also occur during organ/blood transplantation since tachyzoites can infect any tissue. Toxoplasmosis can also be transmitted to the fetus, in which case it is known as congenital toxoplasmosis. Congenital toxoplasmosis can induce death or permanent lesions for the fetus(Saadatnia et al., 2012).
Various other clinical expressions of toxoplasmosis exist depending on the clinical context, the immune status of the patient and the strain of parasite involved. The main clinical pictures are that of primary infection, ocular toxoplasmosis, and toxoplasmosis of the immunocompromised.
Primary infection
Infection in immunocompetent individuals is asymptomatic in the vast majority of cases, even in pregnant women. In 10% of cases, a few non-specific symptoms are observed, but without requiring any particular treatment. However, more serious clinical forms (fevers, headache, asthenia, severe vomiting, eye or more rarely lung damage) may be observed in immunocompetent persons, particularly in the case of infection by certain very virulent strains, most of which are found in Latin America (Dubey et al., 2012).
In immunocompetent patients, due to the mostly asymptomatic nature of the infection and the non-specificity of the symptoms (clinical picture close to CMV, EBV or primary HIV infection), the diagnosis is based on serology. Since the presence of IgG alone does not rule out the diagnosis of an old infection, the diagnosis of primary infection relies on several tests to try to date the infection. In the case of a clinical form, a PCR test for Toxoplasma may be a possible technique.
The determination of a primary infection may be easy if the patient’s serological history is known, or more complex in the case of a first positive sample during pregnancy. In this case, several indicators, all imperfect, can be used: the presence of IgM, the avidity of IgG, the presence of IgA (Olariu et al., 2019), the evolution of the antibody titer (Montoya et al.,2008). The presence of IgM, as most commonly detected by ELISA, is theoretically a marker of primary infection but lacks specificity (Villard et al., 2016a) and residual IgM is detected in a significant number of cases: 1 year after infection between 9 and 27% of patients may still have detectable IgM (Villard et al., 2016b). Avidity of IgG also allows a dating and excludes a recent infection in case of high avidity. However, some patients never develop high avidity so a low avidity does not indicate a recent infection. Finally, a significant increase in IgG levels between 2 samples two weeks apart is in favor of a recent infection. Conversely, stable IgG levels indicate an infection older than 3 months (Villard et al., 2016b).
Because of the mostly asymptomatic nature of the infection, and the difficulties of dating the infection, systematic monitoring of pregnant women during pregnancy, although not carried out in all countries, is a powerful means of preventing congenital toxoplasmosis. For example, in France, the immunological status of pregnant women is established in the first trimester of pregnancy. In case of a negative test, a monthly serology is set up. For cases of seroconversion or suspicion of recent infection, a search for congenital toxoplasmosis must be carried out. Prenatal monitoring for toxoplasmosis is considered a beneficial technique in terms of public health: the cost-benefit ratio is very much in favor of this approach (Prusa et al., 2017), as is the cost-efficacity ratio (Binquet et al., 2019).
Many serological techniques exist (indirect immunofluorescence, ELISA, ISAGA), but the most widespread is ELISA, with specific techniques for IgM and IgG. These techniques have variable performances and require a precise follow-up of the immunological kinetics specific to each patient, particularly as the antibody titers usually measured are very low, with sometimes large grey areas (Villard et al., 2016, Robert-Gangneux et al., 2012).
For an initial diagnosis or for the follow-up of pregnant women, we have developed a rapid and robust test, the Toxoplasma ICT IgG-IgM test. Particularly suitable for first-line laboratories or laboratories with small runs, it is an advantageous replacement for other screening techniques.
In the event of ambiguous or inconsistent results between screening techniques, the French High Authority for Health recommends a confirmatory test by immunoblot or dye-test, the latter technique, which is laborious and non-standardized, being reserved for reference centres (Argumentaire HAS, 2017a).
In order to answer the demand, we have developed a reliable test based on the Western Blot technique. Coupled with the use of highly sensitive and specific natural antigens (T. gondii), the LDBIO Toxo II IgG and LDBIO Toxo II IgM test are thus perfectly positioned as a confirmatory test for IgG and IgM toxoplasmosis serology, even at very low serological levels.
Ocular toxoplasmosis
Ocular toxoplasmosis often occurs in the context of congenital toxoplasmosis. However, particularly in the case of infection by a virulent strain such as those found in South America, ocular toxoplasmosis is also found in the acute phase of infection as well as during reactivation, causing damage to the retina in association with a strong inflammatory reaction. Depending on the location, ocular toxoplasmosis can affect vision or even lead to permanent loss of vision in one or both eyes (Butler et al., 2013).
The diagnostic approach to ocular toxoplasmosis is very specific and is similar to that of congenital toxoplasmosis, of which it is one of the manifestations. It is therefore detailed in the congenital toxoplasmosis section.
Toxoplasmosis of immunosuppressed
In immunocompromised patients, toxoplasmosis can be much more severe. Depending on the cause and intensity of the immunosuppression, several different clinical presentations can be found:
- Primary infection or toxoplasmic reactivation in a patient in advanced AIDS (CD4<100). In this case, the involvement is most often cerebral, taking the form of encephalitis (Vidal, 2019). Toxoplasmosis is one of the main causes of death in AIDS patients. In addition, the HAS in France recommends regular follow-up of HIV-negative patients with toxoplasmosis (Argumentaire HAS, 2017b). For positive patients for toxoplasmosis, and with severely decreased CD4 counts (<100/mm3), Pneumocystis prophylaxis, also active on Toxoplasma, should be offered.
- Positive recipients or positive donor/negative recipient mismatch in the context of solid organ transplantation, in particular cardiac or bone marrow transplantation. In these cases, the risk is that of an acute infection due to the immunosuppression caused by the treatment. This is why the National Reference Centre for Toxoplasmosis in France recommends a serological analysis of toxoplasmosis before transplantation (Villard et al., 2016). The risk is increased for bone marrow and heart transplants (in the case of a positive donor and due to the parasite’s cardiac tropism). In the case of a positive recipient or missmatch, prophylactic treatment must be implemented (Dard et al., 2018).
Due to the humoral weakness of the response in immunocompromised patients, false negative or uninterpretable serologies are common and peripheral blood PCR is the preferred diagnostic test (Villard et al., 2016). However, particularly in the absence of a serological history for an immunosuppressed patient, and in order to avoid the risk of a false negative result, some authors recommend lowering the thresholds for re-testing screening techniques in this population and then confirming with a confirmatory technique (Douet et al., 2020).
In order to answer the demand, we have developed a reliable test based on the Western Blot technique. Coupled with the use of highly sensitive natural antigens (T. gondii), the LDBIO Toxo II IgG test is thus perfectly positioned as a confirmatory test for the diagnosis of toxoplasmosis, even at very low serological levels.
SCIENTIFIC REFERENCES
- Begeman IJ, Lykins J, Zhou Y, Lai BS, Levigne P, El Bissati K, et al. Point-of-caretesting for Toxoplasma gondii IgG/IgM using Toxoplasma ICT IgG-IgM test with sera from the United States and implications for developing countries. 2017; PLoS Negl Trop Dis 11(6): e0005670.
- Douet T, Armengol C, Charpentier E, Chauvin P, Cassaing S, Iriart X, et al. Performance of seven commercial automated assays for the detection of low levels of anti-Toxoplasma IgG in French immunocompromised patients. 2019; Parasite. 26:51.
- Franck J, Garin YJ, Dumon H. LDBio-Toxo II immunoglobulin G Western blot confirmatory test for anti-toxoplasma antibody detection. 2008; J Clin Microbiol.
- Jost C, Touafek F, Fekkar A, Courtin R, Ribeiro M, Mazier D, et al. Utility of immunoblotting for early diagnosis of toxoplasmosis seroconversion in pregnant women. 2011; Clin Vaccine Immunol. 18(11):1908-1912.
- Lykins J, Li X, Levigne P, Zhou Y, El Bissati K, Clouser F, et al. Rapid, inexpensive, fingerstick, whole-blood, sensitive, specific, point-of-care test for anti-Toxoplasma antibodies. 2018; PLoS Negl Trop Dis 12(8): e0006536.
- Mahinc C, Flori P, Delaunay E, Guillerme C, Charaoui S, Raberin H, et al. Evaluation of a new immunochromatography technology test (LDBio Diagnostics) to detect Toxoplasma IgG and IgM: comparison with the routine Architect technique. 2017; J Clin Microbiol 55:3395–3404.
- Maudry A, Chene G, Chatelain R, Patural H, Bellete B, Tisseur B, et al. Bicentric evaluation of six anti-toxoplasma immunoglobulin G (IgG) automated immunoassays and comparison to the Toxo II IgG Western blot. 2009; Clin Vaccine Immunol. 16(9):1322-1326.
- Villard O, Cimon B, L’Ollivier C, Fricker-Hidalgo H, Godineau N, Houze S, et al. Help in the Choice of Automated or Semiautomated Immunoassays for Serological Diagnosis of Toxoplasmosis: Evaluation of Nine Immunoassays by the French National Reference Center for Toxoplasmosis. 2016; J Clin Microbiol. 54(12):3034-3042.
- Villard O, Cimon B, L’Ollivier C, Fricker-Hidalgo H, Godineau N, Houze S, et al. et al. Serological diagnosis of Toxoplasma gondii infection: Recommendations from the French National Reference Center for Toxoplasmosis. Diagn Microbiol Infect Dis. 2016;84(1):22-33.
Our products
Order
To order a product, please send us an email to our address Order with the description of the product. We ship worldwide.
